Expression evaluation of AFP and GPC3 in liver most cancers
To develop efficient and secure CAR-T cell remedy for liver most cancers, we investigated the expression of AFP and GPC3 in hepatocellular carcinoma (HCC) and regular tissues. This evaluation helps predict potential therapeutic results and hostile occasions related to CAR-T merchandise concentrating on these antigens.
We utilized the GEPIA2 database (http://gepia2.cancer-pku.cn), which incorporates gene expression knowledge from The Most cancers Genome Atlas (TCGA). Evaluation of pan-cancer samples revealed important expression of AFP and GPC3 primarily in liver most cancers tissues, with minimal expression in regular tissues and different most cancers varieties (Supplementary Fig. 1A, B and Supplementary Tables 1, 2).
Moreover, we analyzed a cohort of 368 tumor tissues and 160 regular liver tissues from sufferers with HCC. This evaluation confirmed important variations within the expression ranges of AFP and GPC3 between liver most cancers tissue and adjoining non-cancerous tissues (Supplementary Fig. 1C and D).
To strengthen the medical relevance of our findings, we carried out a retrospective evaluation on 65 liver most cancers sufferers identified between 2014 and 2021 at Zhuhai Individuals’s Hospital. Immunohistochemical staining of their pathological specimens revealed that fifty.77% of the sufferers had been optimistic for AFP, 70.77% had been optimistic for GPC3, and 38.46% expressed each antigens (Supplementary Fig. 1E, F, G and Supplementary Desk 3).
These findings strongly recommend that CAR-T cells concentrating on AFP and GPC3, liver cancer-specific antigens, maintain promise for improved remedy efficacy and enhanced security.
Growth of high-affinity AFP-specific antibody
Constructing on the success of our second-generation GPC3 CAR-T with excessive anti-tumor efficacy, we’re initiating the event of an AFP-targeting TCR mimic antibody to discover potential synergistic results together remedy.
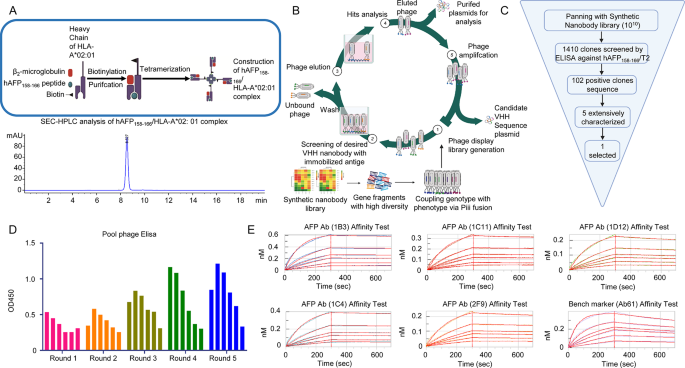
Given the excessive prevalence of the HLA-A*02:01 allele (8.15% globally, 12.04% in China), we targeted on developing the hAFP158 − 166/HLA-A*02:01 complicated for optimum antigen presentation (Fig. 1A Up). Following purification of recombinantβ2m and HLA-A*02:01 proteins, we co-complexed them with the hAFP158 − 166 peptide at an outlined ratio. Using Capto Q ImpRes, we purified the complexes to realize 99.09% purity (Fig. 1A Down).
To establish high-affinity antibodies concentrating on AFP offered by HLA-A*02:01, we employed a biopanning technique on an artificially synthesized phage show antibody library. After 5 rounds of liquid-phase screening, we recognized 5 candidate VHH antibodies (Fig. 1B, C, D). Monoclonal ELISA was used to pick out 5 candidate antibodies exhibiting low background and important signal-to-noise ratios. These candidates had been sequenced and designated as 1B3, 1C11, 1C4, 1D12, and 2F9. Notably, the Ab61 antibody, encoded by the gene described in patent CN107106671A, was synthesized as a optimistic management. Protein A affinity chromatography purified this optimistic management. Subsequent one-step Protein A affinity chromatography and SEC-HPLC evaluation confirmed excessive purity for all VHH antibodies (Supplementary Fig. 2A, B and Supplementary Desk 4).
Lastly, affinity assays had been carried out utilizing a ForteBio AHC sensor-conjugated antibody loaded with every candidate antibody and the hAFP158 − 166/HLA-A*02:01 complicated. All candidate antibodies, besides 1D12, demonstrated superior dissociation constants in comparison with the Ab61 optimistic management. Notably, the 1B3 antibody exhibited an affinity fixed roughly five-fold larger than Ab61 (Fig. 1E and Supplementary Desk 5).
These outcomes spotlight the profitable growth of a high-affinity, particular antibody (1B3) concentrating on hAFP offered by HLA-A*02:01, a vital element for our dual-targeting CAR-T cell remedy technique.
(A) Up, Building means of hAFP158-166/HLA-A*02:01 complicated. Down, Purity evaluation of hAFP158-166/HLA-A*02:01 complicated by SEC-HPLC. (B) Antibody screening course of: ① Synthesis of synthetic nanobody phage show library. ② Screening for humanized VHH antibodies. ③ Elution of phages. ④ Evaluation of goal phages. ⑤ Phage amplification and acquisition of candidate VHH plasmid sequences. (C) The acquisition and screening means of antibodies. (D) 5 rounds of liquid part elution antibody enrichment impact (In every spherical of elution, every column represents a focus gradient of phage inventory answer, which is diluted right into a gradient at a 3-fold ratio from left to proper). (E) Outcomes of affinity assay of various antibodies binding to peptide hAFP158-166/HLA-A*02:01: the affinity of AFP Ab (1B3) antibody reached 0.504nM; the affinity of AFP Ab (1C11) antibody was 1.21nM; the affinity of AFP Ab (1D12) antibody was 3.02nM; the affinity of AFP Ab (1C4) was 0.865nM; the affinity of AFP Ab (2F9) was 2.06nM; the affinity of Bench marker (Ab61) was 2.34nM. The affinity of the three antibodies (1C11, 1D12 and 2F9) was corresponding to the Bench marker (Ab61) (The six curves in Determine E are the fitted curves for the primary six antigen concentrations)
In vitro purposeful analysis of the VHH antibodies for AFP and the related CAR-T
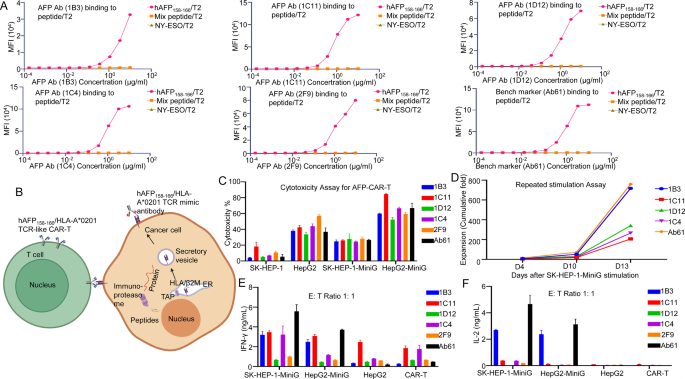
To evaluate the specificity of the VHH antibodies, we carried out binding assays utilizing T2 cells (The T2 Cell is a human lymphocyte hybridoma cell that faithfully presents the goal antigenic peptide to the cell floor, thereby concentrating on the T cell comparable to the goal antibody.), loaded with varied peptide antigens. The outcomes demonstrated that the antibodies exhibited excessive specificity for the hAFP158 − 166/HLA-A*02:01 complicated, with minimal binding to T2 cells loaded with irrelevant peptides (Fig. 2A). Notably, the 1B3 antibody demonstrated robust binding affinity not solely to the HLA-A*02:01 complicated but in addition to the HLA-A*02:07 complicated, suggesting potential applicability in a broader affected person inhabitants (Supplementary Fig. 2C and D).
To judge the cytotoxic potential of the VHH antibodies, we carried out in vitro ADCC assays. The goal cells (HepG2) had been engineered to precise the hAFP158-166/HLA-A*02:01 complicated on their floor (HepG2-MiniG). We co-cultured varied tumor cell traces HepG2 cell (hAFP+/HLA-A*02:01−), HepG2-MiniG cell (hAFP158 − 166+/HLA-A*02:01+), SK-HEP-1 cell (hAFP−/HLA-A*02:01+), and Raji cell (hAFP−/HLA-A*02:01−) and Daudi cell (hAFP−/HLA-A*02:01−) with PBMCs and completely different VHH antibodies. The outcomes confirmed that every one 5 antibodies exhibited particular cytotoxicity in opposition to hAFP158 − 166+/HLA-A*02:01+ tumor cells, with minimal non-specific killing of different cell traces (Supplementary Fig. 3A, B, C, D and E). It’s significantly noteworthy that the AFP Ab (1B3) and AFP Ab (1C11) antibodies didn’t exhibit non-specific cytotoxicity in opposition to SK-HEP-1 cells at any focus (Supplementary Fig. 3D).
To additional examine the therapeutic potential of those VHH antibodies, we engineered CAR-T cells incorporating the VHH sequences. The goal cells (SK-HEP-1 and HepG2) had been engineered to precise the hAFP158 − 166/HLA-A*02:01 complicated on their floor (named SK-HEP-1-MiniG and HepG2-MiniG), enabling recognition and elimination by CAR-T cells (Fig. 2B).
In vitro repeated stimulation experiments demonstrated that 1B3 CAR-T cells exhibited sturdy enlargement and particular cytotoxicity in opposition to goal cells, corresponding to the optimistic management Ab61 CAR-T cells. Furthermore, 1B3 CAR-T cells displayed decrease non-specific cytotoxicity in opposition to hAFP−/HLA-A*02:01− cells, suggesting improved security. Cytokine launch assays revealed that 1B3 CAR-T cells produced related ranges of cytotoxic cytokines as Ab61 CAR-T cells, however with decrease baseline cytokine secretion within the absence of goal cells. This implies that 1B3 CAR-T cells might provide a positive stability of efficacy and security (Fig. 2C, D, E, and F).
These findings collectively reveal the promising potential of the AFP Ab (1B3) antibody and its corresponding CAR-T cells for the focused remedy of liver most cancers, significantly in sufferers expressing HLA-A*02:01 or HLA-A*02:07.
(A) All antibodies confirmed binding exercise to T2 cells loaded with hAFP158-166 peptide, however had non-specific binding to T2 cells loaded with NY-ESO and 19 blended peptides. (B) Illustration of hAFP158-166/HLA-A*02:01 TCR-like CAR-T binding to tumor cells and killing them. (C) In vitro cytotoxicity assay of CAR-T cells constructed with varied antibodies in opposition to completely different most cancers cells (SK-HEP-1, HepG2, SK-HEP-1-MiniG, HepG2-MiniG). (D) The fold enlargement of every CAR-T cell within the in vitro repeated stimulation experiment (goal cells: SK-HEP-1-MiniG). (E) The secretion ranges of IFN-γ by CAR-T cells within the cytotoxicity assay at an effector-to-target ratio of 1:1. (F) The secretion ranges of IL-2 by CAR-T cells within the cytotoxicity experiment at an effector-to-target ratio of 1:1
In vivo efficacy of 1B3 CAR-T cells
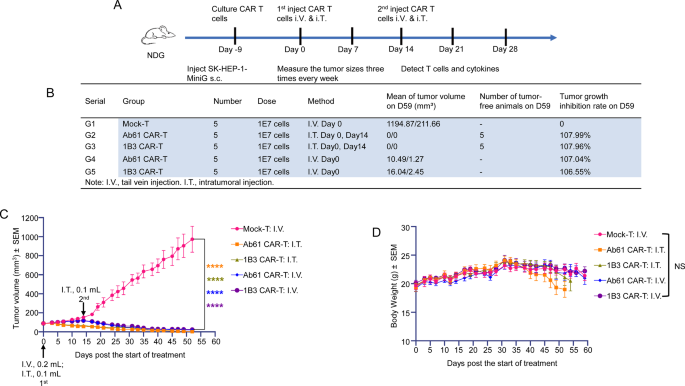
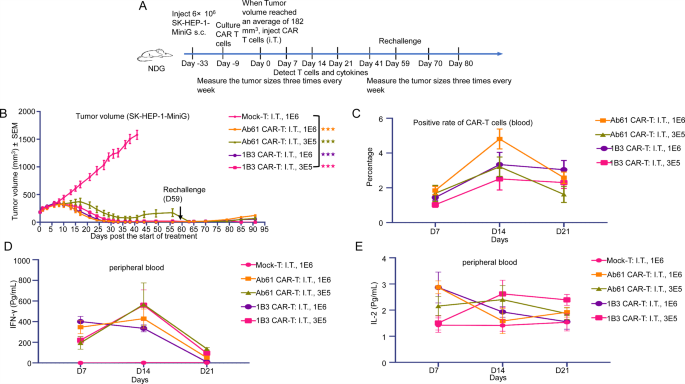
To judge the in vivo efficacy of 1B3 CAR-T cells, we established a subcutaneous tumor mannequin utilizing SK-HEP-1-MiniG cells in mice. Therapy with 1B3 CAR-T cells considerably inhibited tumor progress in comparison with the management group (Fig. 3A, B, and C). Notably, intratumoral administration of CAR-T cells demonstrated enhanced efficacy in comparison with intravenous administration. Importantly, 1B3 CAR-T cells had been well-tolerated, with no important adjustments in physique weight noticed (Fig. 3D).
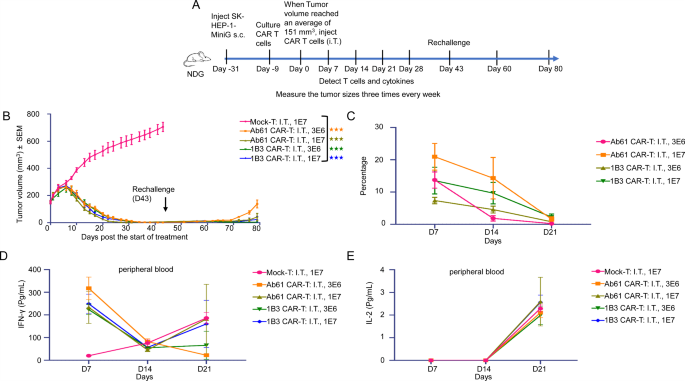
To additional assess the sturdiness of the anti-tumor response, we established a secondary tumor problem mannequin (Fig. 4A and Supplementary Desk 6). Mice had been handled with 1B3 CAR-T cells and, after full tumor regression, had been re-challenged with SK-HEP-1-MiniG cells on the contralateral flank. Remarkably, even at decrease doses, 1B3 CAR-T cells successfully inhibited the expansion of the secondary tumor, demonstrating potent long-lasting anti-tumor exercise (Fig. 4B).
To research the mechanisms underlying the therapeutic efficacy of 1B3 CAR-T cells, we analyzed the persistence and cytokine launch profiles of those cells in vivo. Movement cytometry evaluation revealed that 1B3 CAR-T cells continued within the tumor microenvironment for an prolonged interval, contributing to sustained tumor management (Fig. 4C). Moreover, 1B3 CAR-T cells exhibited a balanced cytokine launch profile, with elevated ranges of IFN-γ and IL-2, essential for efficient tumor cell killing and T cell proliferation, respectively (Fig. 4D, E).
The protection profile of 1B3 CAR-T cells was assessed by monitoring physique weight and peripheral cytokine ranges. No important weight reduction was noticed in any of the remedy teams, indicating good tolerability (Supplementary Fig. 4A). Notably, 1B3 CAR-T cells induced decrease ranges of IL-6, a cytokine related to cytokine launch syndrome (CRS), in comparison with the Ab61 management (Supplementary Fig. 4B). These findings recommend that 1B3 CAR-T cells might have a positive security profile, with a diminished danger of cytokine launch syndrome.
(A) Movement chart of the primary spherical animal experiments (On this research, the primary CAR-T cell remedy injection was administered on Day 0, SK-HEP-1-MiniG had been inoculated in mice on Day − 9, and the second CAR-T cell remedy injection was administered on Day 14. All through the experimental interval, tumor dimension was measured 3 times per week. The proportion of human T cells and cytokine ranges had been assessed by way of peripheral blood testing). (B) Administration strategies and dosages of CAR-T in every group, tumor quantity of mice at endpoint, and tumor inhibition charge. (C) Tumor progress curve of mice in all teams (every group: n = 5). (D) Adjustments in physique weight of mice in every remedy group (every group: n = 5)
(A) Movement chart of the second spherical animal experiments (On this research, SK-HEP-1-MiniG cells had been inoculated subcutaneously in the appropriate flank of mice on Day − 31. When the common tumor quantity reached 151 mm³, CAR-T cells had been administered by way of intratumoral injection. Attributable to important tumor regression, tumor cells had been re-inoculated subcutaneously within the left flank on Day 43 to simulate a tumor recurrence mannequin, aiming to judge the sustained anti-tumor efficacy of CAR-T cells). (B) Tumor progress curve of mice in all teams, rechallenge: mice with tumor regression had been rechallenged with tumor cells on the contralateral aspect to watch the long-lasting tumor-suppressive results of CAR-T (every group: n = 5). (C) CAR-positive charge of CAR-T cells in peripheral blood of mice on day 7, day 14 and day 21 (every group: n = 5). (D) Secretion degree of IFN-γ within the peripheral blood serum of mice on day 7, day 14 and day 21 (every group: n = 5). (E) Secretion degree of IL-2 within the peripheral blood serum of mice on day 7, day 14 and day 21 (every group: n = 5)
Low-dose 1B3 CAR-T remedy: a promising method for most cancers remedy
To additional examine the therapeutic potential of 1B3 CAR-T cells, we carried out a 3rd spherical of in vivo experiments, specializing in low-dose remedy. Mice bearing subcutaneous SK-HEP-1- MiniG tumors had been handled with intratumoral injections of 1 × 106 or 3 × 105 1B3 or Ab61 CAR-T cells (Fig. 5A). Remarkably, even on the low dose of three × 105 cells, 1B3 CAR-T cells demonstrated superior tumor suppression in comparison with Ab61 CAR-T cells (Fig. 5B and Supplementary Desk 7). To evaluate the long-term efficacy, mice had been re-challenged with tumor cells on the contralateral flank. Each 1B3 and Ab61 CAR-T cells successfully suppressed tumor recurrence, highlighting the sturdiness of the anti-tumor response (Fig. 5B).
In distinction to the earlier experiments with larger preliminary tumor burden and CAR-T cell doses, the height in CAR-T cell enlargement and cytokine launch occurred later within the low-dose setting. Particularly, a peak in IFN-γ and TNF-α ranges was noticed on day 14, whereas IL-2 ranges remained elevated all through the research interval (Fig. 5C, D, E and Supplementary Fig. 4D).
Relating to security, no important weight reduction was noticed in any remedy group (Supplementary Fig. 4C). The transient elevation in IL-6 ranges, a cytokine related to cytokine launch syndrome (CRS), was minimal and didn’t persist. These findings recommend that low-dose 1B3 CAR-T remedy affords a positive security profile with minimal hostile results.
General, these outcomes reveal the potent anti-tumor exercise and favorable security profile of low-dose 1B3 CAR-T remedy. This method holds important promise for the remedy of liver most cancers with 1B3 antibody concentrating on AFP.
(A) Movement chart of the third spherical animal experiments (On this experiment, to problem CAR-T cells with bigger tumors, we inoculated SK-HEP-1-MiniG cells subcutaneously on the appropriate flank of mice on Day − 33. When the tumor quantity reached 182 mm³, CAR-T cells had been administered by way of intratumoral injection. Subsequently, speedy tumor regression was noticed. On Day 59, mice had been re-challenged with SK-HEP-1-MiniG cells subcutaneously on the left flank to evaluate the long-term antitumor efficacy of CAR-T cells). (B) Tumor progress curve of mice in all teams, rechallenge: mice with tumor regression had been rechallenged with tumor cells on the contralateral aspect to watch the long-lasting tumor-suppressive results of CAR-T (every group: n = 5). (C) CAR-positive charge of CAR-T cells in peripheral blood of mice on day 7, day 14 and day 21 (every group: n = 5). (D) Secretion degree of IFN-γ within the peripheral blood serum of mice on day 7, day 14 and day 21 (every group: n = 5). (E) Secretion degree of IL-2 within the peripheral blood serum of mice on day 7, day 14 and day 21 (every group: n = 5)
GPC3 CAR-T cells with built-in AFP-CD3 chew: enhanced anti-tumor exercise
Whereas our earlier era of GPC3 CAR-T cells demonstrated promising anti-tumor exercise, we sought to additional optimize their efficacy by incorporating extra concentrating on methods. The combination of an optimized AFP-CD3 BiTE into the CAR-T cell design supplies a dual-targeting method, probably enhancing tumor cell killing and overcoming potential resistance mechanisms.
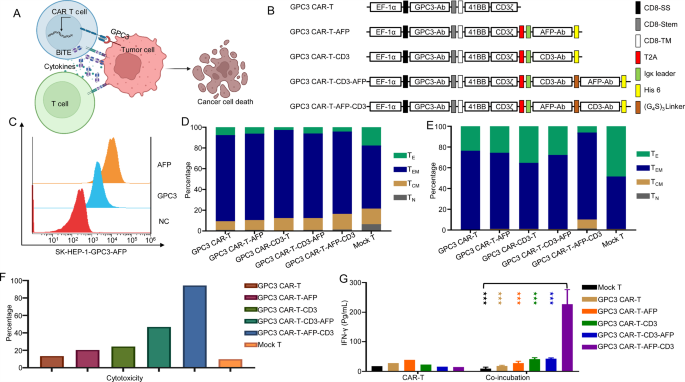
We constructed second-generation GPC3 CAR-T cells incorporating 4-1BB as a co-stimulatory area, built-in an optimized, secreted AFP-CD3 BiTE into the CAR-T cell design. The BiTE molecule, upon secretion, can bind to each AFP-expressing tumor cells and T cells, each endogenous and CAR-T cells, forming an immune synapse and activating bystander T cells (Fig. 6A, B).
To judge the purposeful exercise of those CAR-T cells, we established a goal cell line (Sk-hep-1-GPC3-AFP) overexpressing each GPC3 and the hAFP158 − 166/HLA-A*02:01 complicated (Fig. 6C). In vitro cytotoxicity assays and repeated stimulation experiments demonstrated that GPC3 CAR-T cells with the built-in AFP-CD3 BiTE (GPC3 CAR-T-AFP-CD3) exhibited superior anti-tumor exercise in comparison with single-target GPC3 CAR-T cells (Fig. 6F, G). Moreover, phenotypic evaluation revealed that GPC3 CAR-T-AFP-CD3 cells displayed a blended phenotype of central reminiscence T cells (TCM) and effector reminiscence T cells (TEM) (Fig. 6D, E). This implies that GPC3 CAR-T-AFP-CD3 cells might possess enhanced persistence and long-term anti-tumor exercise. Notably, GPC3 CAR-T-AFP-CD3 cells constantly demonstrated superior efficiency in each cytotoxicity assays and IFN-γ secretion assays (Fig. 6F, G).
These findings spotlight the potential of our dual-targeting technique to enhance the efficacy of CAR-T cell remedy for liver most cancers. By combining direct tumor cell killing with bystander T cell activation, this method might overcome the challenges related to tumor heterogeneity and immune suppression.
(A) Designed and optimized second-generation GPC3 CAR-T cells that incorporate 4-1BB as a co-stimulatory area and are able to secreting BiTE molecules that bind to AFP-expressing tumor cells and T cells. (B) lllustration of GPC3 CAR-T/AFP-BiTE building. (C) Affirmation of GPC3 and AFP expression on track liver most cancers cell line (SK-HEP-1-GPC3-AFP) by stream cytometry. (D) Movement cytometry evaluation was carried out to detect the reminiscence/effector phenotype of CAR T cells. (E) Movement cytometry evaluation was carried out to detect the reminiscence/effector phenotype of CAR T cells after repeated stimulation experiment in vitro. (F) Cytotoxicity of CAR-T Cells in vitro cytotoxicity assay. (G) The secretion ranges of IFN-γ in each cytotoxicity assays and autocrine secretion of assorted CAR-T cells
Mechanistic insights into enhanced efficacy of GPC3 CAR-T-AFP-CD3 cells
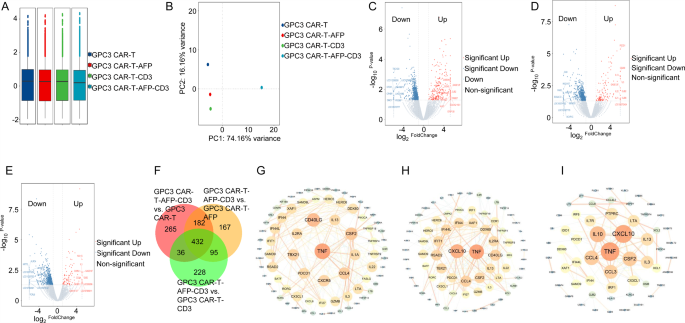
To know the mechanisms underlying the superior anti-tumor exercise of GPC3 CAR-T-AFP-CD3 cells, we carried out transcriptome sequencing on these cells after repeated stimulation. Principal element evaluation revealed distinct gene expression profiles between GPC3 CAR-T-AFP-CD3 cells and management teams (Fig. 7A and B). Volcano plots and Venn diagrams recognized differentially expressed genes (DEGs) throughout teams (Figs. 7C-F and Supplementary Tables 8, 9, 10 and 11). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses revealed enrichment of pathways related to enhanced T cell perform in GPC3 CAR-T-AFP-CD3 cells in comparison with controls (Supplementary Fig. 5A, B, C, D, E and F). Notably, genes like TNF, CD40LG, and CSF2 emerged as key gamers within the purposeful distinction between GPC3 CAR-T-AFP-CD3 and different CAR-T cell variants (Fig. 7G, H and I). These findings recommend that the incorporation of the AFP-CD3 BiTE into GPC3 CAR-T cells (GPC3 CAR-T-AFP-CD3) modulates gene expression, probably contributing to their superior anti-tumor exercise. Additional investigation of those key genes might present useful insights for optimizing CAR-T cell remedy for liver most cancers.
(A) The field plot of gene expression degree. (B) The principal element evaluation of the gene expression of samples in every group. (C) The volcano plot of the general distribution of differentially expressed genes when evaluating the GPC3 CAR-T-AFP-CD3 group with the GPC3 CAR-T group; (D) The volcano plot of the general distribution of differentially expressed genes when evaluating the GPC3 CAR-T-AFP-CD3 group with the GPC3 CAR-T-AFP group; (E) The volcano plot of the general distribution of differentially expressed genes when evaluating the GPC3 CAR-T-AFP-CD3 group with the GPC3 CAR-T-CD3 group. (F) The frequent and distinctive differentially expressed genes amongst completely different comparability teams. (G) The PPI community constructed by differentially expressed genes when evaluating the GPC3 CAR-T-AFP-CD3 group with the GPC3 CAR-T group. (H) The PPI community constructed by differentially expressed genes when evaluating the GPC3 CAR-T-AFP-CD3 group with the GPC3 CAR-T-AFP group. (I) The PPI community constructed by differentially expressed genes when evaluating the GPC3 CAR-T-AFP-CD3 group with the GPC3 CAR-T-CD3 group
In vivo efficacy of GPC3 CAR-T with AFP-CD3 chew
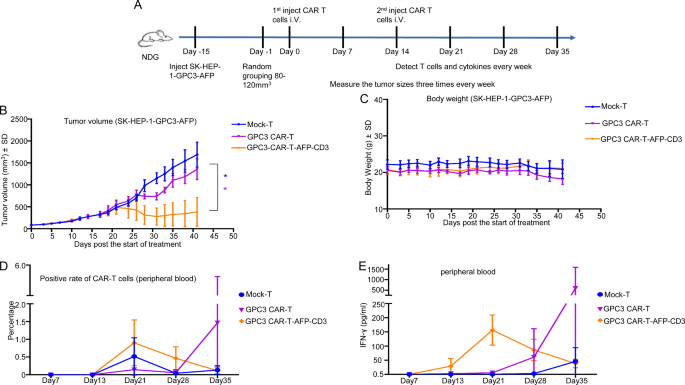
To evaluate the in vivo efficacy of GPC3 CAR-T cells with the built-in AFP-CD3 BiTE (GPC3 CAR-T-AFP-CD3), we established a subcutaneous tumor mannequin in mice (Fig. 8A). When tumor volumes reached 80–120 mm³, mice had been intravenously administered with 1 × 106 or 3 × 106 CAR-T cells. The group handled with 3 × 106 GPC3 CAR-T-AFP-CD3 cells exhibited important tumor progress inhibition and extended survival in comparison with the management teams (Fig. 8B and Supplementary Desk 12). Importantly, no important weight reduction was noticed in any remedy group, indicating a positive security profile (Fig. 8C). Moreover, elevated ranges of IFN-γ within the peripheral blood and enlargement of CAR-positive T cells had been noticed in mice handled with GPC3 CAR-T-AFP-CD3 cells (Fig. 8D, E). These findings recommend that the BiTE-based technique enhances the anti-tumor exercise of CAR-T cells, with the potential recruiting and activating endogenous T cells.
In conclusion, our research demonstrates the potential of GPC3 CAR-T cells with the built-in AFP-CD3 BiTE as a promising therapeutic method for liver most cancers. This dual-targeting technique affords improved anti-tumor efficacy and a positive security profile, making it a promising candidate for future medical translation.
(A) Schematic of the liver most cancers xenograft mannequin used to investicate the in vivo activitof CAR-T cells (On this experiment, SK-HEP-1-GPC3-AFP cells had been inoculated subcutaneously on Day − 15. When tumor quantity reached 80–120 mm³, mice had been randomly grouped. The primary intravenous administration of CAR-T cells was carried out on Day 0, adopted by a second intravenous administration on Day 14). NSG mice had been subcutaneously injected with 3 × 106 goal cells, and CAR-T cells had been injected at specified time factors. Every group consisted of n = 5 mice. (B) Tumor progress was assessed 3 times per week following CAR-T cell injection. (C) ln vivo toxicity was evaluated by monitoring the mice‘s physique weights all through the remedy. (D–E) Peripheral blood samples had been collected weekly to investigate the degrees of CD3+ T cells (D) and IFN-γ (E)